Thousands of deaths each year due to antibiotic resistance – the FFQM promotes the clinical application of personalized phage therapy

As can currently be read in pharmaceutical journals, for example, according to estimates by the Robert Koch Institute and the Institute for Health Metrics and Evaluation at the University of Washington, resistant pathogens are directly responsible for thousands of deaths. The evaluation refers to the year 2019.
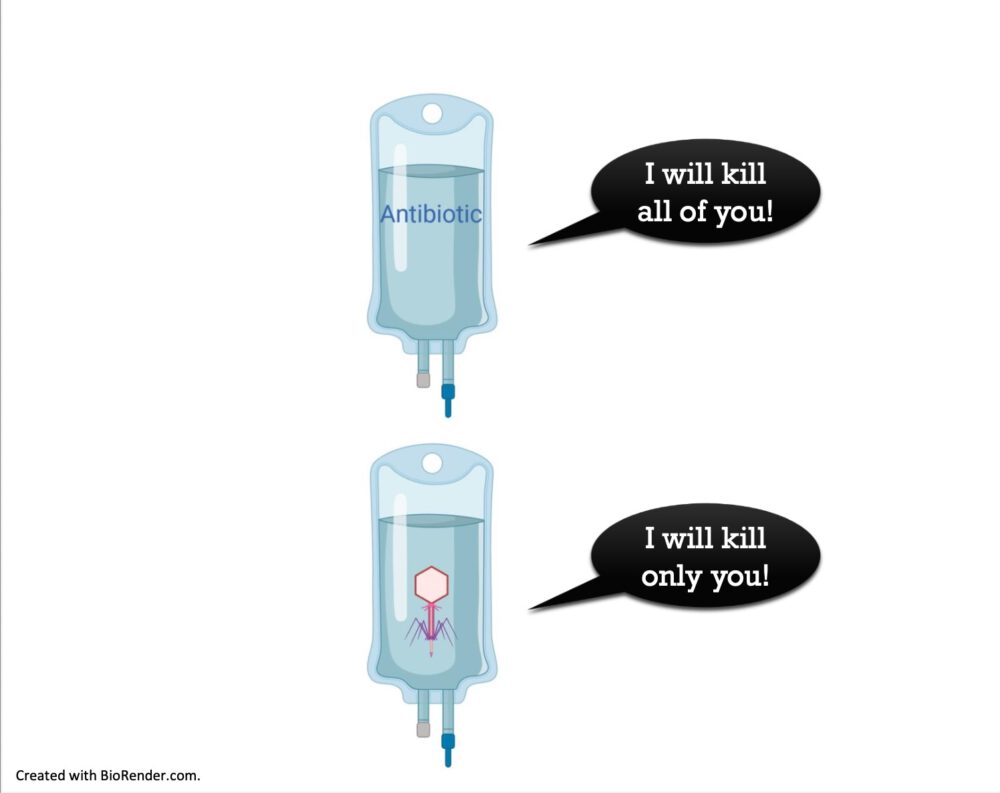
Against the background of the increasing spread of multi-resistant bacterial pathogens, alternative anti-infective therapy approaches such as bacteriophage therapy (phage therapy) are becoming increasingly relevant.
The Frankfurt Foundation Quality of Medicines is therefore pleased to support the PerPhage project, which aims to further develop a quality assurance concept for personalized phage preparations for anti-infective therapy.
Standardized, quality-assured manufacturing processes are required in order to use bacteriophages in a targeted and safe manner. Once these processes have been established, the clinical application of a tailored, safe, and effective complementary phage therapy can be developed for patients in whom antibiotic therapy has failed.
The project pursues two main approaches:
- Optimization of phage purification by improving existing purification processes for the production of a quality-assured API (active pharmaceutical ingredient) using modern chromatographic methods.
- Expansion of quality control methods by developing robust release tests for the pharmaceutical quality control of phage preparations.
The project is led by PD Dr. Silvia Würstle, MBA, Infectious Diseases Section of the II. Medical Clinic at Goethe University Frankfurt Medical Center.
The project fulfills the objectives of the Frankfurt Foundation Quality of Medicines:
- Ensuring and improving drug quality in the field of personalized anti-infective therapies
- Researching innovative processes (innovative API, modern purification, release tests)
- Contributing to the standardization and critical evaluation of quality assurance concepts
- Statewide relevance for the production of phages for personalized therapy as well as for their future clinical application.


